Weak Alkali Examples
Ammonia water ammonium ion hydroxide ion. Ammonium hydroxide may appear to be a non-alkali but its classified as a weak alkali.
Up to 24 cash back A weak alkali only partially dissociates to form OH- ions.
. The conjugate base formed from the deprotonation of formic acid is commonly referred to as formate. Perhaps the most common alkaline material found in the average home sodium bicarbonate is a relatively weak base weighing in at a pH of 83. CONCENTRATION OF AN ACID Text.
NH 3 g H 2 O l NH 4aq OH -aq Some of the ammonia molecules become ions in water. Oranges lemons and limes contain citric acid. They are a subset of bases that contain the alkali metals hydrogen H lithium Li sodium Na potassium K.
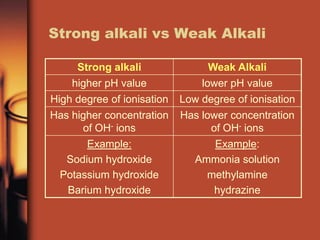
A weak alkali is CHNaO3 also known as baking soda or bicarbonate of soda. Ammonium Hydroxide NH 4 OH Aniline C 6 H 5 NH 2 Ammonia NH 3 Methylamine CH 3 NH 2 Ethylamine CH 3 CH 2 NH 2. A weak alkali has a pH of 11 or 12.
Weak bases are the basic substances that do not completely ionize in water. Alkalis have a special definition in chemistry. NaOH aq Na OH- KOH aq K OH- contains almost - only ions Examples.
All acids taste sour even weak ones like orange juice and all alkalis Bases taste soapy for example. However some ammonia remains unionized in the solution. Bases are divided into two parts Ie strong base and weak base.
On the other hand an acid and a base are weak when they are partially ionized in water that is in solution there will be a proportion of cations another proportion of anions and. When a weak acid and a weak base are mixed they come to an equilibrium state represented by the following equation. A weak alkali is only partly less than 100 ionised.
An example of a weak base is ammonia. List of Weak Bases. Many weak bases are simply slightly soluble hydroxides like magnesium hydroxide while others are organic compounds.
The conjugate base of a weak acid is a weak base while the conjugate acid of a weak base is a weak acid. Alkalis are very corrosive in nature and penetrate deeply. How does a weak acid taste.
Alkalis are soft in nature as they have weaker metallic bond. Examples of weak alkalis are. An example of a strong alkali is is sodium hydroxide.
Updated on January 29 2020. When NH 3 is dissolved in water a part of it dissociates into ammonium cation and hydroxide anions by interacting with the water molecules. Examples of strong alkalis lyes include barium sodium ammonium calcium lithium and potassium hydroxides.
Acid and Bases are distinguished by its nature of aqueous solution. Vinegar is diluted acetic acid which is what gives salad dressings and pickled vegetables their tart taste. When it dissociates in water it does not release many of its OH- ions.
Examples of acids in everyday life. By contrast a strong alkali like NaOH sodium hydroxide or caustic soda releases almost all of its OH- ions in solution. It has been said that all alkali are bases but not all bases are alkali.
If a weak acid or weak alkali is used or both are weak the standard enthalpy of neutralisation is generally less exothermic. Strong Alkali Is an alkali which dissociates almost completely in aqueous solution thereby producing a high concentration of hydroxyl OH ions. This weak acid is known to form a miscible mixture with water.
Go to STRENGTH VS. An acid and a base are strong when they are completely ionized that is in the ionization process they are completely transformed into cations or positive ions and into anions or negative ions. An alkali is a basic hydroxide which when dissolved in water produces hydroxyl ions OH- as the only negatively charged ions.
The equilibrium position lies on the side of the weaker acid and the weaker base and we can determine whether the resulting solution is acidic basic or neutral by comparing the Ka and Kb values of these species. What is an example of a weak alkali. However some weak bases such as ammonia are weak electrolytes.
The term of base or alkaline is derived from the Arabic Al Qali which means Abu. Bases are often paired with acid as both are mutually neutralizing. The pK a of this weak acid is equal to 3745.
This is because heat energy is required to ionise a weak acid or base. An example of a weak alkali is ammonia. Examples of bases and alkalis in everyday life.
A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. Pg63 Mechanical pulp bleaching 21 48-51 chelation in 21 49 new developments in 21 50-51 using weak alkali sources in 21 50-51 Mechanical. HAaq Baq Aaq HBaq.
The melting point of formic acid is 84 degrees celsius whereas the boiling point of this compound corresponds to 1008 degrees celsius. But most of them stay as molecules. Anne Marie Helmenstine PhD.
Examples of Weak Base 5 Examples with Images - Teachoo Chemistry. In contrast a strong acid fully dissociates into its ions in water. Alkali is a term for water-soluble base.
The ionic alkalis NaOH and KOH are essential alkalis. What are alkalis examples. This is because its soluble in water and contains hydrogen.
Strong bases can be soluble in water whereas weak bases do not. Examples of Strong Base and Weak Bases Solutions. Examples of an Alkali.
Here is a list of some weak base examples. Up to 24 cash back 1-3 is a Strong Acid 4-7 is a Weak Acid 7 is Neutral 8-10 is a Weak Alkali 11-14 is a Strong Alkali. Powered by Create your own unique website with customizable templates.
Lithium Sodium and Potassium hydroxide Weak alkali Is an alkali which dissociates only partially in aqueous.

List Of Strong Bases 7 Examples Chemistry Teachoo
Difference Between Weak Base And Strong Base Difference Between

Strong And Weak Alkali S Acids Bases Alkali S Chemistry Fuseschool Youtube
Comments
Post a Comment